Equivalent sp2 orbitals, leaving one p orbital untouched The process is shown below 2s 2p X 2p y 2p z Potential energy sp2 hybridization sp2 sp2 sp2 p In this top view, the unhybridized p orbital cannot be seen because it also arranges itself to be as far apart from the sp2 orbitals as possible That is to say, it is positioned at rightSP Flash Tool (SmartPhone Flash Tool) helps you flash or install the Stock Firmware (scatter based) on your Mediatek powered Smartphone and Tablet It also allows you to flash a custom recovery, Format the Device, Reset the Device and Remove FRP Protection Use any of the following SP Flash Tool to flash or install the firmware (ROM) on your Mediatek DeviceS&P Global Sign In New User Sign Up Use your existing S&P Global login credentials to access Marketplace Otherwise, please enter your email address below to begin the registration process You may gain access to this product if your current company has an active Master Subscription Agreement with S&P Global Market Intelligence
Which One Of The Following Statements Is False A An Chegg Com
Sp one pte ltd
Sp one pte ltd-Make Google your default search provider in your browser to get the fastest access to Google Search results Switching is fast and easySp2 hybridization in ethene In sp^2 hybridization, the 2s orbital mixes with only two of the three available 2p orbitals, forming a total of three sp^2 orbitals with one porbital remaining The two carbon atoms form a sigma bond in the molecule by overlapping two sp 2 orbitals Each carbon atom forms two covalent bonds with hydrogen by s–sp




Ethiopia Learning Chemistry Grade 11 Page 173 In English
Remarks Use sp_change_users_login to link a database user in the current database with a SQL Server login If the login for a user has changed, use sp_change_users_login to link the user to the new login without losing user permissions The new login cannot be sa, and the user cannot be dbo, guest, or an INFORMATION_SCHEMA userThe Standard and Poor's 500, or simply the S&P 500, is a stock market index comprised of 500 large companies listed on stock exchanges in the United States It is oneAs if you have more than one pension or annuity, a working spouse, or a large amount of income outside of your pensions After your Form W‐4P takes effect, you can also use this estimator to see how the amount of tax you're having withheld compares to your projected total tax for 21 If you use the estimator, you
S&P 500 PE Ratio chart, historic, and current data Current S&P 500 PE Ratio is 34, a change of 003 from previous market closeIn sp hybridization, one s orbital and one p orbital hybridize to form two sp orbitals, each consisting of 50% s character and 50% p character This type of hybridization is required whenever an atom is surrounded by two groups of electronsDow Jones The Dow Jones branded indices are proprietary to and are calculated, distributed and marketed by DJI Opco, a subsidiary of S&P Dow Jones Indices LLC and have been licensed for
@pnp/sp¶ This package contains the fluent api used to call the SharePoint rest services Getting Started¶ Install the library and required dependenciesP&G Ventures Among Top Best Workplaces for Innovators Read more Engage, Act, and make an Impact Read more Delivering on Diversity Read moreNeck pain imposes a considerable personal and socioeconomic burdenit is one of the top five chronic pain conditions in terms of prevalence and years lost to disabilityyet it receives a fraction of the research funding given to low back pain Although most acute episodes resolve spontaneously, more




Chemistry The Central Science Chapter 9 Section 5
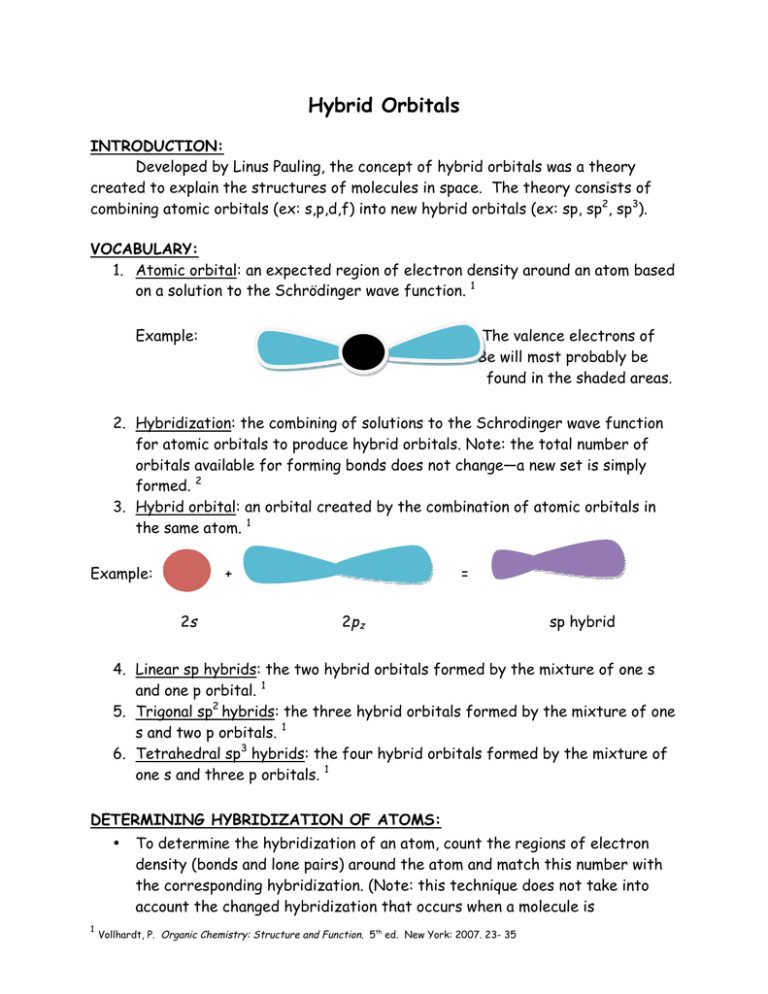



Hybrid Orbitals
SAP ONE Support Launchpad What would you like to do next? The PSOne console was one of the most popular game systems of all time PlayStation 1 games were produced by hundreds of Sony PlayStation game developers The PlayStation One games featured state of the art graphics The PlayStation 1 now has a cult following amongst classic game collectorsSp 3 Hybridization When one 's' orbital and 3 'p' orbitals belonging to the same shell of an atom mix together to form four new equivalent orbital, the type of hybridization is called a tetrahedral hybridization or sp 3 The new orbitals formed are called sp 3 hybrid orbitals




Poland S Pzl Gull Wing Fighters Volume One P 1 Through P 8 Warren A Eberspacher Jan P Koniarek Amazon Com Books



First Lessons In Zoology Zoology Fig 36 Rarainq Fiiiiii P Buccal Groove At Right Greatly Magnified From Life Fk 37 L Oiiiit A Sp One Individual With Stalk Coiled And One With Stalk
Each sp hybrid orbital has one large and one small lobe The combination of one s and two p orbitals will form a group of three hybrid orbitals These hybrid orbitals adopt a(n) planar geometry and are at an angle of o to the remaining unhybridized p orbitalAn sp^3 orbital is unsymmetrical in shape, having one small and one large lobeThe four sp^3 hybrid orbitals of a group are equivalent in shape and energy Select all the statements that correctly describe hybridization involving d orbitalsthe hybridization of one s, three p and one d orbital gives five sp3d orbitalsWith VSP, your vision care comes first We're committed to providing you with the best choices in eye doctors and eyeglasses, all while saving you hundreds!




Abarth 1000 Sp Is An Alfa Romeo Powered One Off



P S Rcd113 3 Switch Single Pole 3 Way Sp Sp Gordon Electric Supply Inc
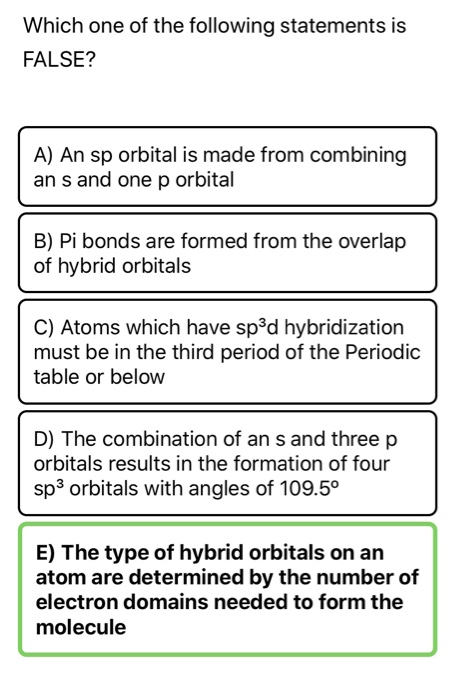
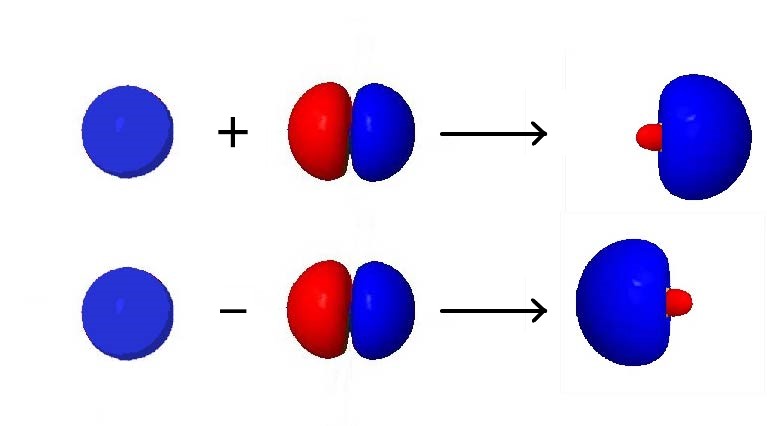
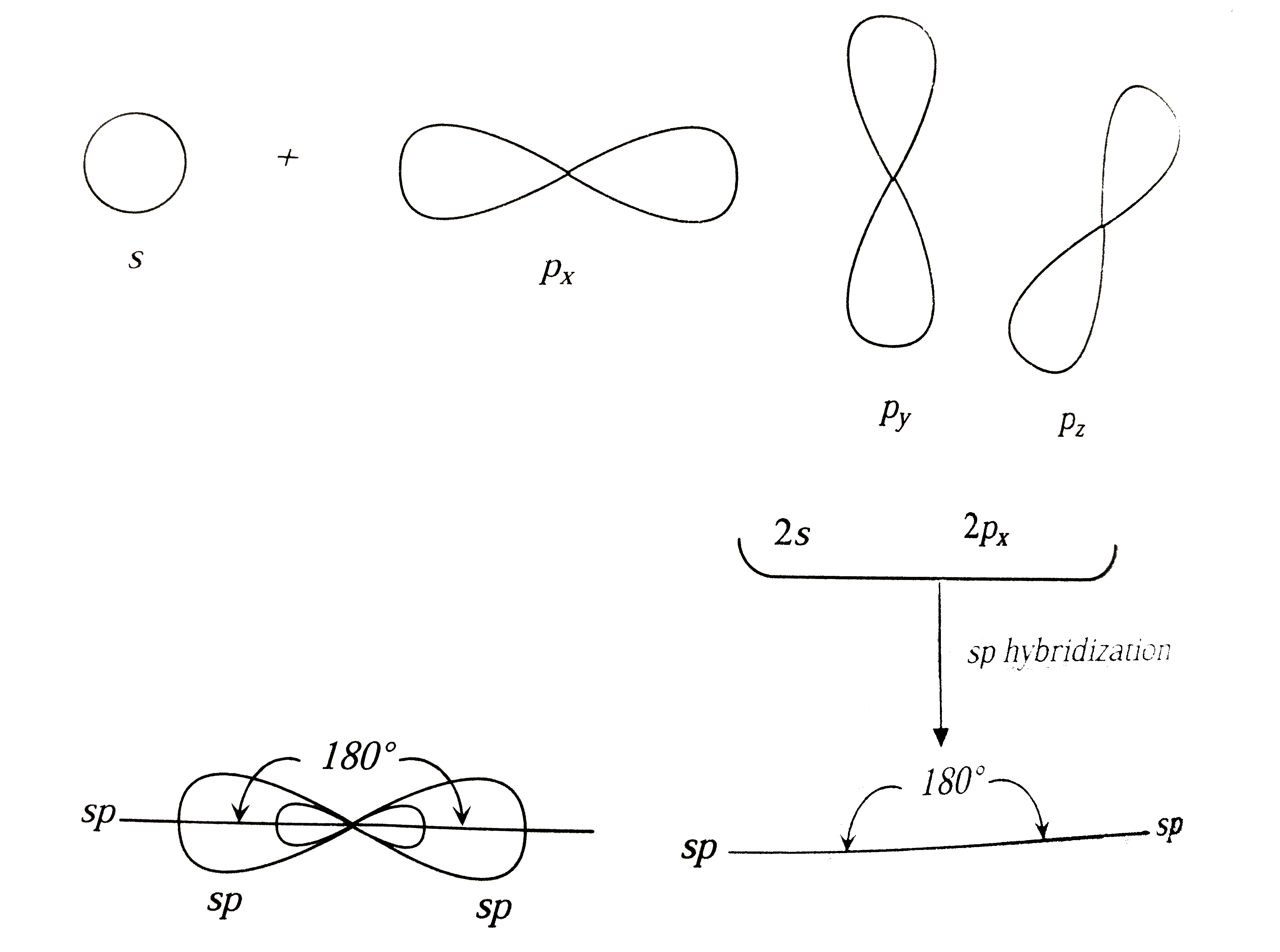
A) An sp orbital is made from combining ans and one p orbital B) Pi bonds are formed from the overlap of hybrid orbitals C) Atoms which have sp°d hybridization must be in the third period of the Periodic table or below D) The combination of an s and three p orbitals results in the formation of four sp³ orbitals with angles of 1095° E) TheYearend statements can be obtained from our website after February 10th, 21 Log into your account to view or print a copy of your statement If you have been affected by a natural disaster, we are here to help We recommend that you first file a claim with your insurance company, then call us at so we can assist you sp Hybridization sp Hybridization can explain the linear structure in molecules In it, the 2s orbital and one of the 2p orbitals hybridize to form two sp orbitals, each consisting of 50% s and 50% p character The front lobes face away from each other and form a straight line leaving a 180° angle between the two orbitals



キャンペーン 新品未使用 Celsus Sound Sp One P パッシブタイプ 2本1組 スピーカー セルサスサウンド Spone P Celsus Spone P モバックス 通販 Yahoo ショッピング




Sp One P Celsus Sound Hifi Do Mcintosh Jbl Audio Technica Jeff Rowland Accuphase
In sp² hybridization, one s orbital and two p orbitals hybridize to form three sp² orbitals, each consisting of 33% s character and 67% p character This type of hybridization is required whenever an atom is surrounded by three groups of electronsCurrent and Historical Performance Performance for SPDR S&P 500 on Yahoo FinanceWhich one of the following statements is FALSE?




One S P French Mc And Dj In Edinburgh Scroll Down Menu Here Blog Archive Naked Circle Jam Toastie Tailor One S P Jed 104 Prod Dj Red6 Out Now




Ethiopia Learning Chemistry Grade 11 Page 173 In English
Sp 2 Hybridization The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp 2 hybrid orbitals and one unhybridized p orbital This arrangement results from sp 2 hybridization, the mixing of one s orbital and two p orbitals to produce three identical hybrid orbitals oriented in a trigonal planar geometry ()The North American Aviation P51 Mustang is an American longrange, singleseat fighter and fighterbomber used during World War II and the Korean War, among other conflictsThe Mustang was designed in April 1940 by a design team headed by James Kindelberger of North American Aviation (NAA) in response to a requirement of the British Purchasing CommissionBest LowLatency Data Feed Provider;



Molecular Formulas And Nomenclature




In Sp Hybridization The 2s Orbital And A 2p Orbital Hybridize To Form Two Sp Orbitals Each Consisting Of 50 S Cha Organic Chemistry Chemistry Chemical Bonds
SP Richards connects top suppliers, dealers and retailers Offering comprehensive warehousing & distribution services Dropshipping from a nationwide network of 33 centers, annually handling over 25 million cartons! S&P Asia 50 6, 2% S&P China 500 3, 057% S&P 500 Bond 003% S&P 500 VIX ShortTerm 126 105% IndexThe unhybridized atomic p orbital lies at a 90° angle to the plane This configuration allows for the maximum separation of all orbitals Last, the atomic orbitals of carbon can hybridize by the linear combination of one s and one p orbital This process forms two equivalent sp hybrid orbitals The remaining two atomic p orbitals remain




Masters Of None 0th Level Characters Dungeon Masters Guild Dungeon Masters Guild




Which One Of The Following Statements Is False A An Chegg Com
SP 961 PLATE 9 OF 14 Selected References, Layton sand and Nellie Bly Formation w; When one s and two p orbitals of the same shell of an atom mix to form 3 equivalent orbitals, the type of hybridisation is called sp 2 hybridisation The new orbitals formed are called sp 2 hybrid orbitals All the three hybrid orbitals remain in the same plane making an angle of 1° with oneFind the latest information on S&P 500 (^GSPC) including data, charts, related news and more from Yahoo Finance




Lecture 16 C 1403 October 31 05 18




Amazon Com Roland Xc 540 Sc 540 Sp 300 P Roller Td16s4 Type2 One Couple Us Stock Office Products
About S&P 500 INDEX The S&P 500® is widely regarded as the best single gauge of largecap US equities and serves as the foundation for a wide range ofUse mouse wheel to zoom in and out Drag zoomed map to pan it Double‑click a ticker to display detailed information in a new window Hover mouse cursor over a ticker to see its main competitors in a stacked view with a 3month history graph sp 2 Hybridization The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp 2 hybrid orbitals and one unhybridized p orbital This arrangement results from sp 2 hybridization, the mixing of one s orbital and two p orbitals to produce three identical hybrid orbitals oriented in a trigonal planar geometry (Figure




Sphynx Sp One Sp Three Kuantokusta




Two Oocysts Of Eimeria Sp One Viable And Sporulating On The Left Download Scientific Diagram
A)An sp orbital is made from combining an s and one p orbital b)Pi bonds are formed from the overlap of hydrogen orbitals c)Atoms which have sp3d hybridization must be in the third period of the Periodic Table or below d)The combination of an s and three p orbitals result in the formation of four sp3 orbitals with angles of 1095 e)TheIn sp hybridization, the s orbital overlaps with only one p orbital Any central atom surrounded by just two regions of valence electron density in a molecule will exhibit sp hybridization sp orbitals are oriented at 180 degrees to each other2s 2p sp 3 (ex) NH 3 N 2s 2p sp 3 (ex) H 2 O O (5) sp 3 d hybrid orbital • one s orbital three p orbitals one d orbital • trigonal bipyramidal 3s 3p 3d (ex) PCl 5 P 3d sp 3 d (6) sp 3 d 2 hybrid orbital • one s orbital three p orbitals two d orbital • octahedral 3s 3p 3d (ex) SF 6 S 3d sp 3 d 2




Porcelanosa Sp One Xl Bathtub Tiles And Bathrooms Online




Bookshelf Speakers Review Shootout Celsus Sound Sp One Vs Centrance Masterclass 2504 Vs John Blue Jb3 Headphone Reviews And Discussion Head Fi Org
If your company has a current subscription with S&P Global Market Intelligence, you can register as a new user for access to the platform(s) covered by your license at Market Intelligence platform or S&P Capital IQ23 23 N 24 29 22 MISSOURI H 350 E 15 o o w G 16 A E G M o G s o s N c M A M G s N R N G G G F D R E R A D H E E N o LAYTON SAND AND NELLIE BLY FORMATION Paden area, Lincoln and Okfuskee Counties, Oklahoma ShaleSP 971 P 6TIF Author murr5940 Created Date 12/1/10 PM




Monsoon Mmrs End Cap Mmrs Sp 1p With Two Side Ports And One End Port Titan Rig



2
Celsus Sound SPONE/P 小型ブックシェルフ型スピーカー NEW 定価60,000円(ペア/税別) 形式 : 2ウェイ・バスレフ型 搭載ユニット ・高域 : ソフトドーム型 ・低域 : 35インチ・カーボンファイバー・コーン 周波数特性 : 50Hz~kHzOpen SAP ONE Support Launchpad Visit SAP Support Portal or SAPcomRecent awards include Best Credit Risk Management Product;




Pinterest Osterreich




One Piece T Shirt Men Unlimited Cruise Sp Japan Anime The Straw Hat Pirates Luffy Tshirt Summer Cotton Shirts Black Short Sleeve T Shirts Aliexpress
UNITYD Storage File After one SP reboots, the NAS server on that SP takes too long to fail over to the peer SP UNITYD Storage File Sometimes the SP rebooted unexpectedly when a NAS server or SP was in the process of failing over UNITYD145/ UNITYD Storage File During an autoshrink or repurpose operation on the fileGo to the Windows 7 Service Pack 1 download page on the Microsoft website Select Install Instructions to see which packages are available for download, and make note of the one that you need Select the appropriate language from the dropdown list, and then select Download Select the packages you need to install, select Next, and then followFor the hydrogen fluoride molecule, for example, two F lone pairs are essentially unhybridized p orbitals, while the other is an sp x hybrid orbital An analogous consideration applies to water (one O lone pair is in a pure p orbital, another is in an sp x hybrid orbital) See also Crystal field theory;




Suite In One By Walter Mertens Steve Weiss Music



2



Annotationes Zoologicae Japonenses Nihon Do Butsugaku Iho New Or Imperfectly Known Species Of Earthworms 15 P Shimaensis N Sp One Specimen Preserved In Formol Length 5 Mm Breadth 7 Mm



Apple Gate Project Bushwick Gorey Sp One Whisper Quik Binho




Sp Hybridization Youtube




What Are Sp And Sp 2 Hybridization How Do They Differ From Sp 3 Quora




Aerie Swimsuit One Piece S P Pink Swimsuit One Piece Long By Aerie Size S P No Bad Waves Aerie Swim One Pieces Aerie Swimsuits One Piece Swimsuit One Piece




One O Clock Samba By Lynn Glassock Steve Weiss Music



Plos One A New Diatom Species P Hallegraeffii Sp Nov Belonging To The Toxic Genus Pseudo Nitzschia Bacillariophyceae From The East Australian Current




Simple Plan Di Twitter Vip Pizza Party Upgrades For The Pop Punk S Still Not Dead Tour Are On Sale Now At T Co Nqczbtj19z We Re So Excited To Finally Be Back On Stage Playing




Sp3 Sp2 And Sp Hybridization In Organic Chemistry With Practice Problems Chemistry Steps




1 Chemical Bonding 2 Lewis Theory An Overview Valence E Play A Fundamental Role In Chemical Bonding E Transfer Leads To Ionic Bonds Sharing Of Ppt Download




Buy Class A Customs T 1300 Sp One 1 Spouted 13 Gallon Rv Fresh And Gray Water Holding Tank Rv Concession Online In Netherlands B01bjt5e



Controlling Stereochemistry During Oxidative Coupling Preparation Of Rp Or Sp Phosphoramidates From One P Chiral Precursor Chemical Communications Rsc Publishing




Amazon Com Dragon Ball Super Tcg One Hit Destruction Vegeta Sp Set Promo Cards Ball Super Cards P 001 Toys Games




オーディオスクェア藤沢店のブログ 話題の小型スピーカー Celsus Sound セルサス サウンド Sp One P を期間限定でお聴き頂けます




Sp One P Celsus Sound Hifi Do Mcintosh Jbl Audio Technica Jeff Rowland Accuphase




Alterations Of Substance P Sp Levels At Superficial Laminae Of L5 S2 Download Scientific Diagram
:format(jpeg):mode_rgb():quality(90)/discogs-images/R-5294101-1593810293-4489.jpeg.jpg)



Nolan Thomas One Bad Apple 1985 Sp Specialty Pressing Vinyl Discogs




Alterations Of Substance P Sp Levels At Superficial Laminae Of T2 T5 Download Scientific Diagram




Celsus Sp One Active Speakers Review Headfonics Com



Plos One Expression Of A Chimeric Antigen Receptor In Multiple Leukocyte Lineages In Transgenic Mice




Sp One P Celsus Sound Hifi Do Mcintosh Jbl Audio Technica Jeff Rowland Accuphase



送料無料 メーカー在庫限り Celsus Sound Sp One A アクティブタイプ 2本1組 スピーカー セルサスサウンド Sponea 2 売れ筋 Www Dmaa At




Spone P Kitiona 684kitiona Twitter




Valence Bond Theory Electrons Are Not Simply Dots




Megahouse P O P X Pinkyst Street Nami One Piece Figure




Monkey Salt And Pepper Balancing Act One Pc Set Monkey S P




Federal Premium Punch 38 Sp P Jhp 1gr Bop Limit One Per Week In Store Pick Up Only Northwest Armory



Dreer S Garden Book Seventy Sixth Annual Edition 1914 Seeds Catalogs Nursery Stock Catalogs Gardening Equipment And Supplies Catalogs Flowers Seeds Catalogs Vegetables Seeds Catalogs Fruit Seeds Catalogs Steckrube Ger J



Www Parasound Com Pdfs Vintage Psp1000om Pdf




Sp One P Celsus Sound Hifi Do Mcintosh Jbl Audio Technica Jeff Rowland Accuphase



Answer With Counter Claim S Jam Pharmacy Corp Trellis




Styles P S P The Goat Ghost Of All Time Digipak Criminalatl




Skirted One Piece Dual Flush 3 4 5l Washdown Toilet With P Trap 453x Sp Kohler



D10 5 Types Of Hybrid Orbitals Chemistry 109 Fall 21



送料無料 メーカー在庫限り Celsus Sound Sp One A アクティブタイプ 2本1組 スピーカー セルサスサウンド Sponea 2 売れ筋 Www Dmaa At




B Sf C Sc14 1 Sp D No L P 2 Sp One L P 3 Sp One




Expression Of Substance P Sp In The Human Adrenal Gland A Download Scientific Diagram




Buy Spd One Percussion Sam Ash Music



Plos One Expression Of A Chimeric Antigen Receptor In Multiple Leukocyte Lineages In Transgenic Mice



More Marineford Characters Join One Piece Unlimited Cruise Sp Siliconera




New Power One Xyratex Sp Pcm2 He764 Ac Power Supply Unit Psu 764w 04



1 15 Bonding In Methane And Orbital Hybridization Pdf Free Download




Becca By Rebecca Virtue Green Swimwear Sausalito Bandeau Monokini Halter S P One Piece Bathing Suit Size 4 S Tradesy



One Hybridization Of One S And One P Orbital We Get




新品未使用 Celsus Sound Sp One P パッシブタイプ 2本1組 スピーカー セルサスサウンド Spone P 携帯 スマホの激安販売通販ショップ モバックス Mobax 新品 中古
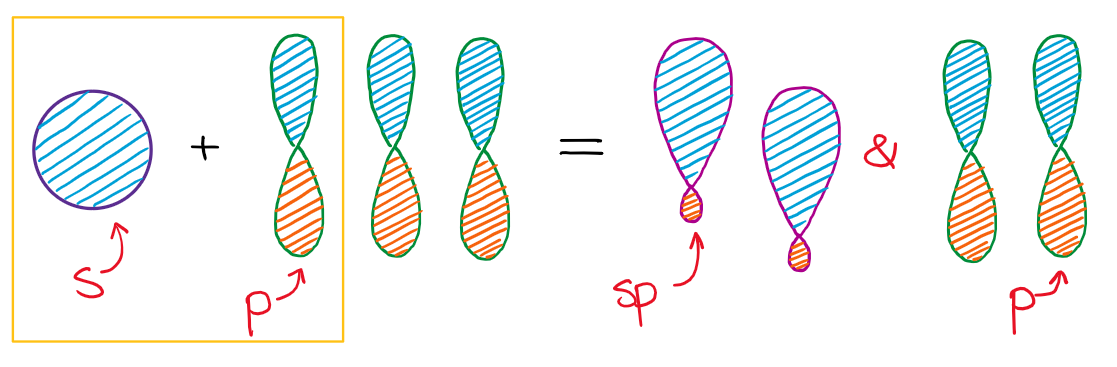



Hybridization Organic Chemistry Tutor



After Almost One Year Of Playing Superstar Bts I Finally Got Sp P For Spring Day Superstarbts




1992 Marvel The Mcfarlane Era P 3 Number One Spider Man Prism Sp In Hard Case Ebay




Review Celsus Sound Sp One P 좋은 소리를 만들겠다는 의지가 가득 담긴 작은 스피커 네이버 블로그




Vistosi Neochic Sp S Minimal Chandelier Light Shopping



Baby Silicone Plate Bibs Bowl Spone Feeding Tableware Waterproof Non Slip Crockery Bpa Free Silicone Dishes For Baby Bowl Baby P Dishes Aliexpress




Kate Spade Claretta P S 0581 Sp Havana Black Rectangular Sunglasses Sunsvision Com



Hybridization Department Of Chemistry




Bel Artsp Scienceware Single Piece Buchner Funnels Funnels Filtering Funnels Fisher Scientific



Niceproduce Com P 5959




Chapter 9 Molecular Geometries Lamar University Texas Pages 51 58 Flip Pdf Download Fliphtml5




Wall Socket Adapter For Sp C2s3a Charger Nuvair



One World Sp Youtube




Sp One P Celsus Sound Hifi Do Mcintosh Jbl Audio Technica Jeff Rowland Accuphase
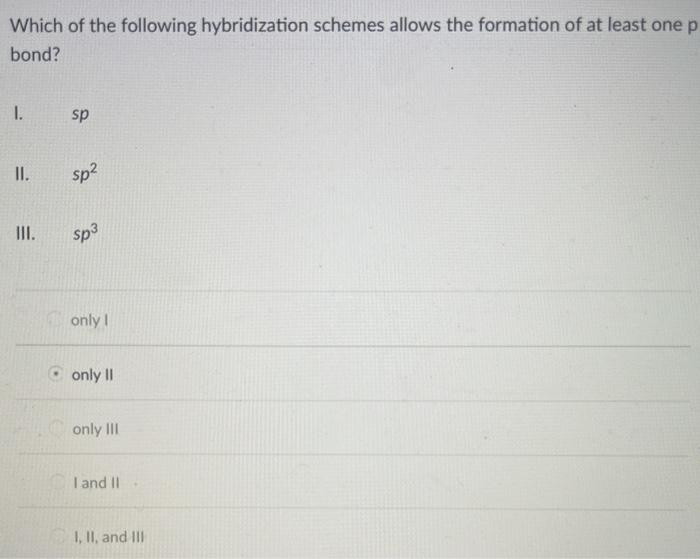



Solved Which Of The Following Hybridization Schemes Allow Chegg Com



Gray Anchor Roma 10a S P One Way Switch Module Size 1 Module 230 V Rs 30 Piece Id



Page 3 Sellerie High Resolution Stock Photography And Images Alamy



Plos One Seed Biopriming With P And K Solubilizing Enterobacter Hormaechei Sp Improves The Early Vegetative Growth And The P And K Uptake Of Okra Abelmoschus Esculentus Seedling




Dwimmerlaik Volume 1 Issue 1 September 1968 Tolkien Fanzines Marquette University E Archives




One Man S Junk By W J Putnam Steve Weiss Music



Boston 2crsi Octopus 1 4sp One Node And Four Gpu




Sp One Piper Pa 34 2t Seneca Iii Private Bogdan Jankowiak Jetphotos
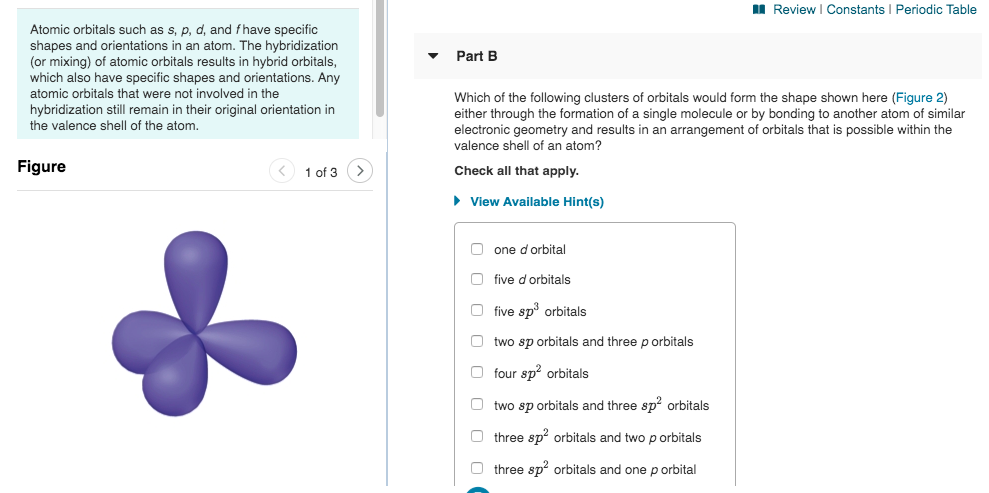



1 Review Constants Periodic Table Part B Atomic Chegg Com
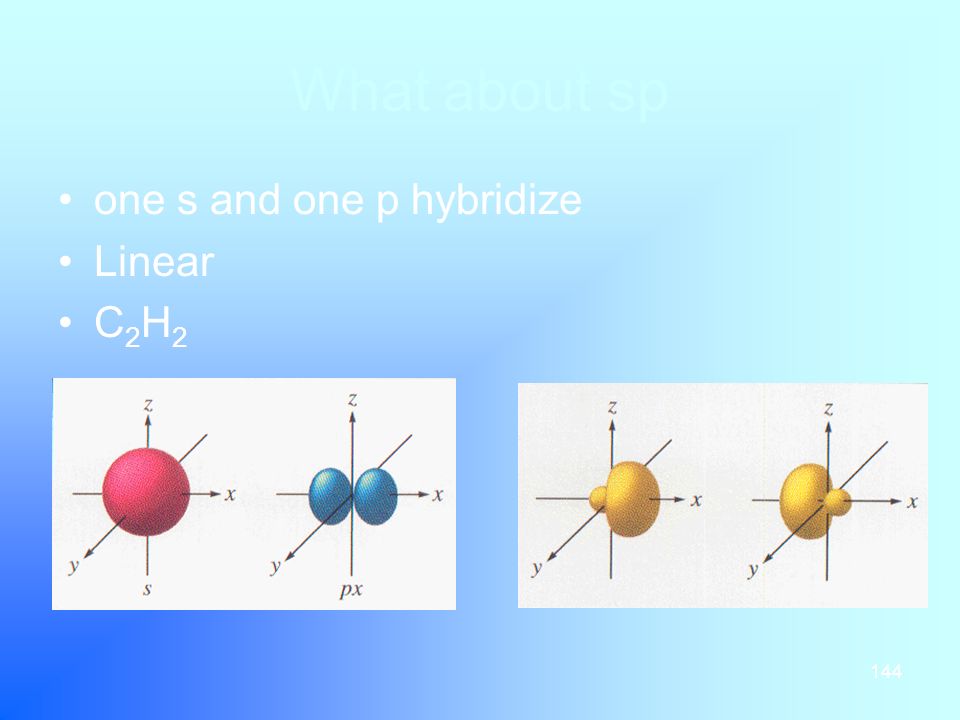



Chapter 7 Chemical Bonding Ppt Download




Winchester 30 06 180 Gr Power Point Sp One Full One Partial 35 Rounds




Ch9z5eorbitalsunhidden Phpapp02




Sp One Photos Facebook
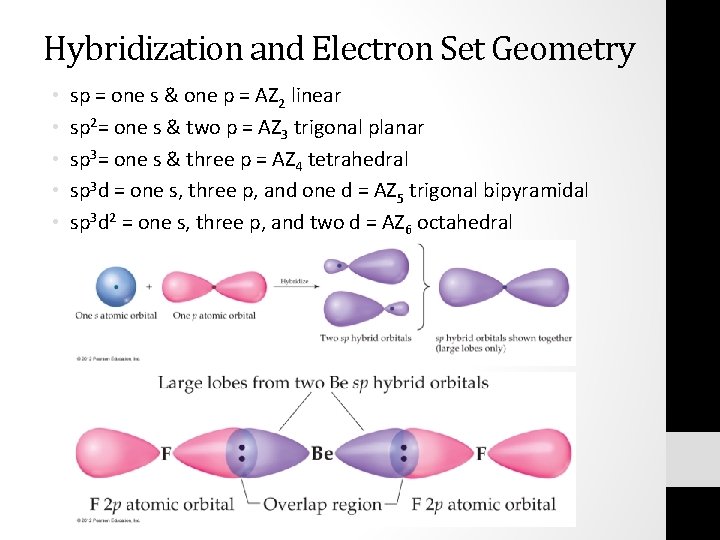



Chapter 9 Notes Ap Chemistry Galster Molecular Geometry




One Sp Controls 501 001spc Sp2 Chassis Pcb Working For Sale Online Ebay



0 件のコメント:
コメントを投稿